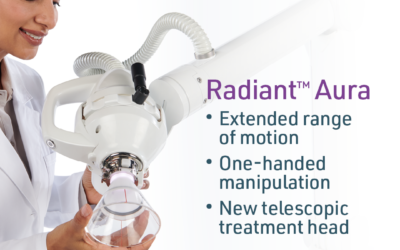
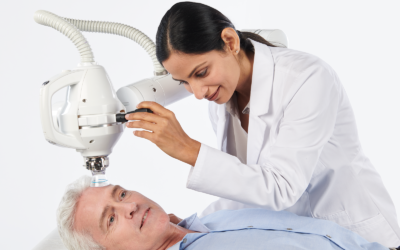
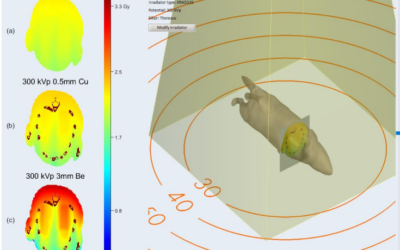
Xstrahl, a global leader in the delivery of superficial radiation therapy devices and preclinical radiation research systems, will demonstrate Radiant Aura at the Maui Derm Hawaii 2024 meeting, January 22-26, 2024. Radiant Aura is a new low-dose, X-ray based, dual modality radiation therapy for treating non-melanoma skin cancers (NMSC) and keloid scarring.
News
Contact Us