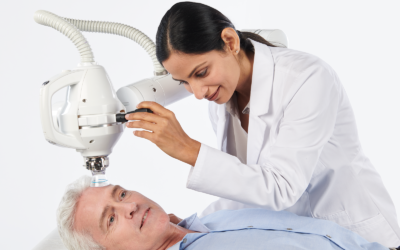
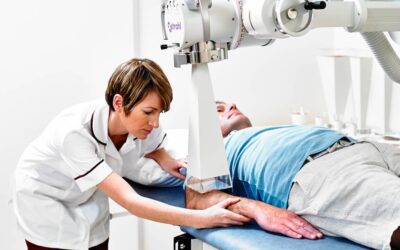
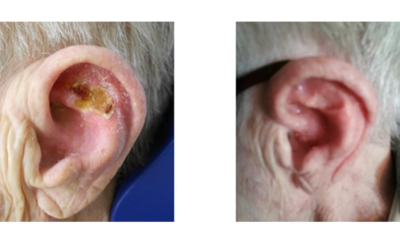
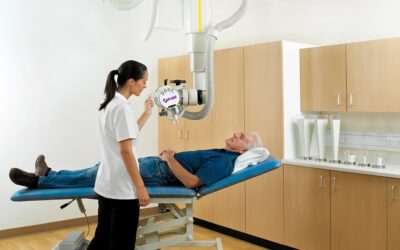
Xstrahl, a global leader in the delivery of superficial radiation therapy devices and preclinical radiation research systems, will demonstrate Radiant Aura at the CalDerm 2024 NorCal Conference at the Meritage Resort & Spa from May 3-5 in Napa, CA.
Resource Type
Contact Us