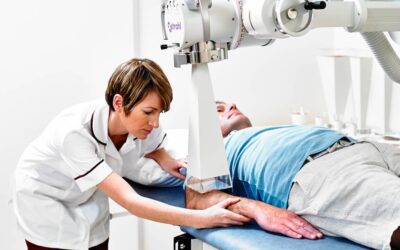
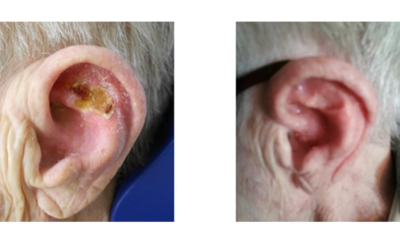
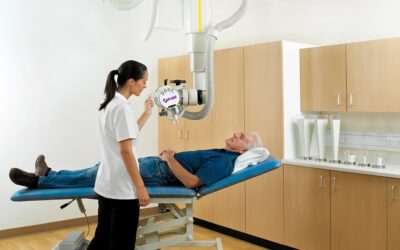
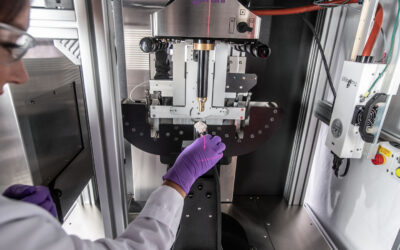
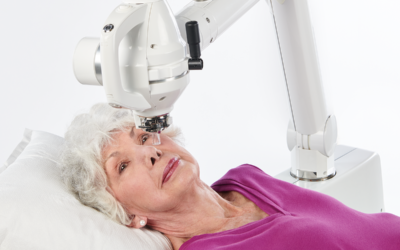
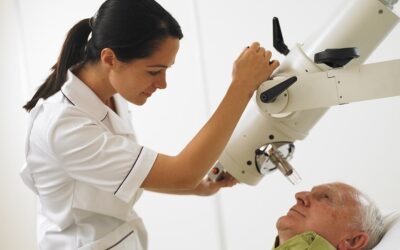
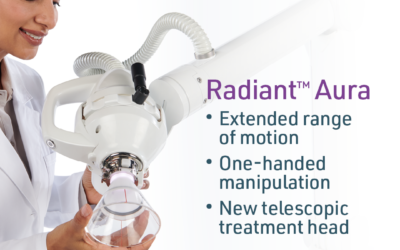
Xstrahl, a global leader in the delivery of superficial radiation therapy devices and preclinical radiation research systems, will demonstrate Xstrahl 200, a modern orthovoltage radiotherapy solution for treating superficial lesions, skin cancers, and selective benign conditions at the European Society for Radiotherapy and Oncology (ESTRO) Annual Meeting in Glasgow, UK, May 3-7, 2024, stand # 1070.
Resources
Contact Us