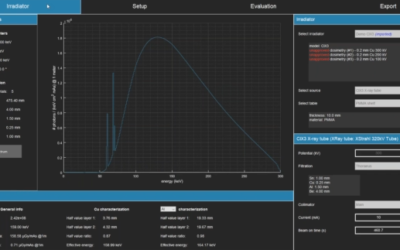
SmART-XPS is one of the Treatment Planning System options for our Small Animal Radiation Research Platform (SARRP), alongside MuriPlan. Both systems have a wide range of features and capabilities. Learn more about SmART-XPS below.
XBeam V2: FDA-Approved Treatment Planning Software
The Fastest, Safest Way to Plan Superficial & Orthovoltage Treatment XBeam V2 is the revolutionary, FDA 510(k)-cleared dose planning software designed to automate planning, eliminate risk, and seamlessly integrates with the Concerto system, Xstrahl’s...