The mission of most cancer centers, in some shape or form, is to contribute to the prevention and cure of cancer. To accomplish such a mission, a large focus must be placed on research in both the clinical and pre-clinical laboratory setting. In order to yield clinically relevant results, clinically relevant research must be done. Therefore, it is imperative to perform the most accurate and translational research to achieve the mission of the institution. In order to uphold the mission, most laboratories and cores facilities make available to their researchers highly specific, technical, and analytical systems like HPLC, Mass Spec, Flow Cytometry, Fluorescence/ Bioluminescent Imaging, Nuclear Magnetic Resonance, and Confocal Microscopy. However, the available systems ability to accurately model radiation delivery in small animals is lacking.
For the majority of cancer centers and cancer based research institutes. The current status of radiation based research utilizes very basic and simplistic methodology to deliver radiation to desired areas when working with small animal models. Through conversations with radiation oncologists, radiobiologists, physicists, and cancer researchers of all backgrounds. I have begun to understand that the systems currently available in the pre-clinical setting are limited to fixed source, single planar cabinet irradiators. These irradiators do not allow for beam collimation, do not have imaging capabilities, and require the researcher to use lead cutouts/jigs to shield areas not desired to be treated while leaving the target (tumor) exposed to the radiation. To most investigators this sounds just fine, mainly because it is how radiation delivery has been modeled in small animals for a very long time. However, many uncontrolled variables, assumptions, and lack of reproducibility are a result of this type of modeling. The reason is these systems lack the ability to image, treatment plan, visualize and measure dose distribution, accurately target in space, and minimize normal tissue toxicity. Which are routinely performed practices in the clinic to treat patients with cancer. Over 60% of patients are treated with some form of radiation during their treatment for cancer, therefore it is important to model clinical practice accurately. It is my conclusion that the current methods of delivering radiation in small animal modeling (both flank and orthotopic) at most research institutions are not clinically translational.
Recently, I had an at length conversation with an investigator that will remain unnamed for privacy concerns. During this discussion, he described the current animal modeling in his project to be bi-flank breast or melanoma subcutaneous tumors investigating abscopal effect in response to radiation. The modeling is designed around shielding the animal and adjacent tumor with lead, and exposing the tumor to radiation in a single plane. This type of modeling will allow investigators to gain baseline insight into cellular and molecular interactions responding to radiation, however the data is hardly translational to clinical practice. Many unknowns in this model exist, for instance what dose is the tumor receiving? At no point are you able to measure or visualize if the dose is conformal to the tumor. Furthermore, dose scatter that is occurring from the lead, and subsequent normal tissue exposure cannot be measured or accounted for. This can result in unforeseen experimental variables as normal tissue exposure to radiation can skew abscopal effect considerably. This model is being developed to generate data to support a mathematical model which will calculate treatment response in humans. If the data generated isn’t done so with methods that are performed clinically, I would hesitate to translate those results to indication of human response. The benefit of SARRP can be seen in a study by Dilworth et al., titled Preclinical Models for Translational Research Should Maintain Pace With Modern Clinical Practice (IJROBP, 2013). This study highlights the SARRPs ability to deliver multiple beams from different angles compared to a single planar cabinet system. It was observed that mice treated with SARRP had better local tumor control, increased survival, and were able to experience dose escalation to 60Gy (clinical Tx schedule of 2Gy/day) compared to 30Gy when using a cabinet.
Clinically relevant research yields clinically relevant results. It is impossible to achieve this with outdated and inaccurate methods of radiation delivery to small animal cancer models. To contribute to the prevention and cure of cancer, cancer researchers must be performing clinically translational radiation based research. The importance of such methodology and effectively translating these results to impact clinical practice is covered extensively in a review titled “Current Clinical Trials Testing Combinations of Immunotherapy and Radiation” (Crittended et al., Seminars in Radiation Oncology, 2015). These techniques are easily performed and achieved by utilizing a system called SARRP (Small Animal Image Guided Micro-Irradiator), otherwise known as a 3D image guided micro- irradiator (3D IGMI).
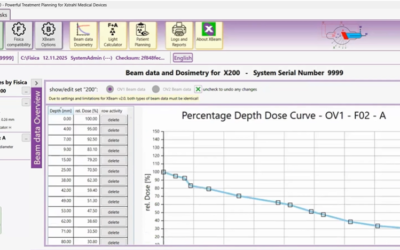
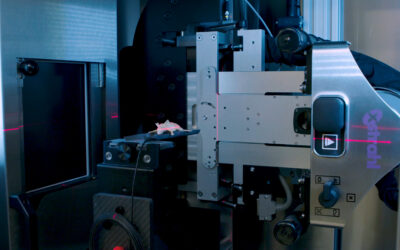
SARRP allows pre-clinical investigators to mimic clinical radiation practice. SARRP is a fully shielded X-ray system that allows for the delivery of a beam(s) of radiation from any angle. The system consists of a 360° rotating gantry, 20cm x 25cm high resolution CT panel, 225 kVp X-ray tube, bed rotation in X, Y, Z, and Θ allowing for non-coplanar beam arrangements, dynamic field size dictation, cone beam-CT imaging, and a clinically relevant treatment planning software (MuriPlan). The SARRP is accurate within 0.2mm of isocenter, and can deliver clinically relevant beam arrangements such as continuous arcs, static beams, cranial spinal irradiation, and SBRT. With on board cone beam-CT imaging, the superfast GPU reconstructs the image in real time (60-70 seconds) so there is no wait between acquiring the CT and reconstruction. Once the CBCT is finished, the image is easily uploaded into MuriPlan which takes you through a series of modules allowing for image registration (DICOM files from MRI or PET), contouring, isocenter placement, beam arrangement, dose verification, and execution. Furthermore, MuriPlan is a fully integrated software that allows full control of all hardware components in the SARRP that are associated with treatment planning and execution.
SARRP was originally developed by Dr. John Wong at John’s Hopkins University as a system to mimic clinical radiation practice in small animal cancer modeling. Since then, Xstrahl Inc. has commercially developed SARRP in collaboration with the group at Johns Hopkins. With over 100 publications to date in peer reviewed journals, and 61 SARRP worldwide at institutions like Dana Farber Cancer Institute, University of Arkansas Medical School, Oxford, Columbia, University of Pennsylvania, St. Judes, and University of California San Francisco. SARRP is the leading 3D image guided micro-irradiator being integrated into single labs, and core facilities to be used as a shared resource.
Since most large-scale cancer research institutes now have diverse core facilities that offer technologies to help in accomplishing their mission, SARRP can easily be integrated into the already established infrastructure. This will allow investigators from various cancer based backgrounds to utilize a technology which will drive their research forward, publish in high impact journals, and generate translational data. Furthermore, it allows clinical mindsets to be adapted in a preclinical setting, and for clinical trial validation as was done in Radiation and Dual Checkpoint Blockade Activate Non-Redundant Immune Mechanisms in Cancer (Twyman-Saint Victor et al., Nature, 2015). Integrating SARRP into the already established facilities will only benefit the investigators and institution, allowing for further support of the mission to contribute to the prevention and cure of cancer
This Xstrahl In Action was adapted from a article found on a National Library of Medicine website.