Throughout the last 100 years, numerous milestones in the study and treatment of cancer have been achieved. Discoveries like combination chemotherapy, correlation of smoking and lung cancer, HPV and cervical carcinogenesis, BRCA-1&2 gene inheritance and breast carcinogenesis, and fractionated radiation therapy have allowed oncologists to be more effective at successfully treating the disease. But wait, there’s more (insert Billy Mays thumbs up)! That’s right folks, a bit over 100 years ago a man by the name of Paul Ehrlich presented a concept known as “immune surveillance”. In a nutshell, the hypothesis stated that the immune system has the capability to suppresses tumor formation. Starting to sound a bit familiar? Probably so because over the last 20 years or so the world has witnessed the reemergence of immune surveillance, or better known today as immunotherapy.
The two most characterized (clinically and pre-clinically), and now FDA approved immunotherapeutic drugs include anti-PD1 and anti-CTLA4. Of the two, anti-PD1 has received the most attention, and you’ve likely seen it marketed for clinical use as either Pembrolizumab or Nivolumab, manufactured by Merck and Bristol Myers Squibb, respectively. Unfortunately, not all patients respond positively to anti-PD1 and/or anti-CTLA4. Because of this, further investigation and understanding of the immune response which stimulates anti-tumor cells are necessary, especially in poorly immunogenic tumors. Furthermore, radiation (varying fractionation regimes) in combination with immunotherapy has shown to enhance the immune checkpoint effectiveness, benefit tumor response, and stimulate anti-tumor T cells.
To further understand the dynamic relationship between radiation fractionation and immunotherapy in cancer, a group led by Dr.’s Sandra Demaria and Silvia Formenti at Weill Cornell Medicine have identified the DNA exonuclease Trex1 as a culprit in modulating tumor immunogenicity. The study published in Nature Communications in June of 2017, titled “DNA exonuclease Trex1 regulates radiotherapy-induced tumor immunogenicity” identifies optimal radiation doses and fractionation with SARRP, specific DNA signaling pathways, T-cell response, and effects of Trex1 when stimulated or antagonized (Vanpouille-Box et al.)
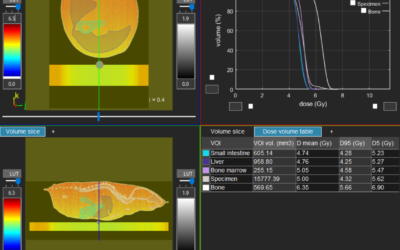
In order to identify how radiation interacts with anti-CTLA4 to stimulate anti-tumor T-cells, the authors developed a bi-flank abscopal tumor model using a poorly immunogenic mammary mouse carcinoma, TSA (Figure 1a, Vanpouille-Box et al.) Here, they were able to show that a dose of 8Gy x 3 + anti-CTLA4 results in a positive abscopal response when compared to anti-CTLA4 alone, 8Gy, 8Gy + anti-CTLA4, 30Gy, 30Gy + anti CTLA4, or 3 x 8Gy (Figure 1b). These data suggest the benefit of fractionation compared to large high doses of radiation.
Since Trex1 has previously been shown to degrade damaged DNA which accumulates in the cytosol after high doses (12-18Gy), ultimately mitigating any immune response mitigated through STING, cGAS/GAMP, and IFN-β expression. A set of experiments quantifying the expression of the Trex1 as an upstream regulator of anti-CTLA4 after lower fractionated doses, 8Gy x3, was performed to understand if fractionation could exploit the immune response. Not only was 8Gy x 3 shown to cause more DNA damage than other doses investigated, the expression of Trex1 was also significantly reduced (Figure 2a-b). Furthermore, the expression of IFN-β, a key component in stimulating the recruitment and activation of dendritic cells which are responsible for priming anti-tumor T cells was also significantly increased (Figure 2c). These results, as well as other findings in the publication, suggest 8Gy x 3 as an optimal dose regimen to exploit a Trex1 mediated immune response.
Through their observations of 8Gy x 3 combined with anti-CTLA4 and the expression of Trex1, the authors developed an abscopal model which investigated these combinations together. It was observed that the induction of Trex1 through the addition of doxycycline completely abrogated the tumor response in both the irradiated and non-irradiated sites for both anti-CTLA4 (Figure 3a-b) and anti-PD1 (Figure 3c-d).
Further data expressed in the full manuscript showed the benefit of 20Gy x 2 when Trex1 was knocked down, further suggesting the benefit of a fractionated treatment regimen to enhance tumor response. Together these data suggest the benefit in combination treatments focused on fractionation and drug delivery in poorly immunogenic tumors. The authors conclude that Trex1 should be considered as an attractive target for interventions to improve in situ vaccination by radiotherapy and by other DNA damaging targets (Vanpouille-Box et al.)
As much as the scientific community and world population would like there to be a silver bullet to cure all cancer, that likely does not exist. What does exist, however, is the vast understanding by groups like the one led by Dr.’s Sandra Demaria and Silvia Formenti of how multiple pathways cross-talk and communicate with one another, similar to what is shown in this manuscript. The capability of these investigators to understand the synergy between treatment, signaling pathways, and tumor response combined with Xstrahl’s capacity to provide cutting-edge radiation technology like SARRP, that is the silver bullet. There isn’t going to be one specific tool in the shed, but the combination of using all the tools in the shed to complete the job, to eradicate cancer.
The full manuscript can be downloaded at https://www.ncbi.nlm.nih.gov/pubmed/28598415
Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature Communications. 2017
This Xstrahl In Action was adapted from a article found on a National Library of Medicine website.