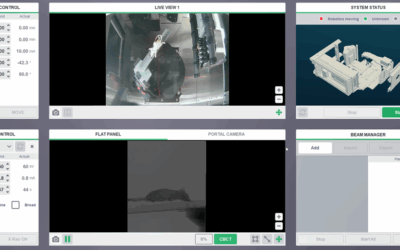
Preclinical radiation biology has become increasingly sophisticated due to the implementation of advanced small animal image guided radiation platforms into laboratory investigation. The Xstrahl small animal radiation research platform (SARRP) enables state-of-the-art image guided radiotherapy research to be performed by combining high-resolution cone beam computed tomography imaging with an isocentric irradiation system. This platform is capable of replicating modern clinical systems similar to those that integrate a linear accelerator with on-board CBCT image guidance. To achieve clinically relevant data, clinically relevant biological research AND quality assurance must be performed to ensure precision and accuracy.
Quality assurance via dosimetric procedures for small animal image guided micro-irradiators originate from the medical physics codes of practice used by clinical radiotherapy departments. These practices normally incorporate specific corrections for low energy beams and backscatter for broad field exposures. However, the most important difference between clinical and pre-clinical dosimetry are the utilized field sizes: while dosimetry for stereotactic small fields involves Gafchromic films and thermoluminescent detectors for areas under 0.8 × 0.8 cm2, preclinical dosimetry employs even smaller fields.
In radiobiology, very small and precise radiation beams (soft X-ray and charged particles) have long been used to deliver radiation to specific subcellular compartments. However, as dosimetry, these tools mostly use different particle counters to calculate the exact energy delivered to the targeted cells.
This study shows the full commissioning of SARRP, including physical, focusing on the 0.5 mm diameter apertures. Small size apertures are intended to be used for a very precise beam delivery. The present work aims to elucidate the specific technical aspects of the small beam use in preclinical radiobiology. The beam characterization and determination of the absorbed dose has been performed according to the AAPM TG-61 code of practice.
Overall, a good agreement (1.7–3%) was observed when comparing the measured physical doses and the commissioning data provided by Xstrahl for all apertures used. Furthermore, all small field dosimetry data were similar for both film reading methods (Matlab and FilmQA), and with Monte Carlo simulations for both focal spot sizes (Broad: 5.5mm and 220kV/13mA, Fine: 1.0mm and 220kV/3mA). It was observed that the fine focal spot produced a more homogenous beam with more stable penumbra over time. However, it is important to know that when a dose delivered with the fine focal setting, a decrease in dose rate occurs. Therefore, the time to deliver a specific dose to the target will take longer than a broad focal spot, but the beam quality will be better. It is important to choose the electron beam with care as this can potentially impact the beam stability and reproducibility.
Since SARRP was commercialized, a main focus of Xstrahl has been to be involved in ongoing research that further characterizes SARRP and pushes the experimental envelope. This is made in part by sponsored research, and collaborations with institutions who share a common experimental goal and mindset. Recently, our friends at Queen’s University in Belfast, Ireland suggested a dosimetric validation study which would further characterize SARRP field homogeneity. Through collaboration of Dr. Jonathan Kane and Amanda Tulk of Xstrahl Inc., and Dr. Mihaela Ghita et. al of Queen’s University, a study investigating small field dosimetry with SARRP was forged. The study was published in Radiation Oncology in late 2017 and was presented this year AACR in Chicago.
This Xstrahl In Action was adapted from a article found on a National Library of Medicine website.