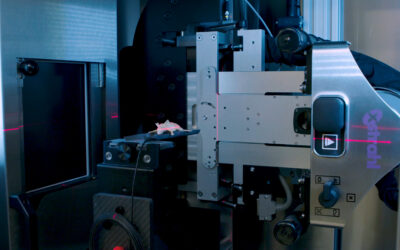
At the 2017 Annual Meeting of the American Society for Radiation Oncology (ASTRO) researchers announced the development of an efficient and reproducible method to deliver craniospinal irradiation to patient-derived xenograft in mouse models.
Despite aggressive multimodality therapy with high-dose craniospinal irradiation and adjuvant chemotherapy, primary therapy fails in a significant number of high-risk patients.
The purpose of the preclinical trials designed by Martine F. Roussel, PhD, and his colleagues from St. Jude Children’s Research Hospital, Memphis, Tennessee to more faithfully recapitulate aspects of the human disease and therapeutic interventions for high-risk medulloblastomas. The ultimate goal is to evaluate novel combination therapies systematically.
The investigators established and molecularly characterized patient-derived xenografts of high-risk group 3 medulloblastoma and Sonic hedgehog medulloblastoma subgroups through stereotactic implantation into the cortexes of CD1 nu/nu mice.
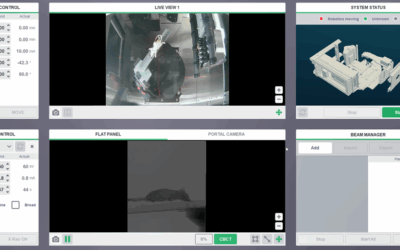
Fractionated whole brain radiation therapy was delivered with an orthovoltage irradiator (2 Gy daily to 36 – 40 Gy), while the Xstrahl Small Animal Radiation Research Platform (SARRP) equipped with a variable collimator was utilized to deliver image-guided, fractionated craniospinal irradiation. Daily-dose-volume histograms were obtained in a subset of brain tumor-bearing mice through Xstrahl’s MuriPlan software.
Tumor-bearing mice were followed for survival metrics, and tumor burden was tracked with weekly bioluminescent imaging using the IVIS Xenogen System. Body weight, complete blood counts, and liver enzymes were monitored on a weekly basis to evaluate treatment toxicity. Central nervous system tissue samples were collected for histopathologic analysis.
Whole brain radiotherapy significantly prolonged the survival of mice bearing patient-derived xenografts with high-risk group 3 medulloblastoma (HDMBO3, P = .0016; ICB1572, P = .0086) and a Sonic hedgehog medulloblastoma patient-derived xenograph model (TB13-5634, P = .0309).
Tumors ultimately developed in central nervous system regions shielded from radiotherapy, including the olfactory bulb and spine.
The SARRP was utilized to develop an optimal craniospinal treatment plan composed of an arc cranium field and a two-field spine approach. The plan enabled a mean brain V95% of 98.3% ± 1.6% and mean spine V95% of 88.0% ± 7.6% over the course of craniospinal irradiation, while limiting the dose to the upper aerodigestive tract and bowel.
Craniospinal irradiation (36 Gy) prolonged the survival of mice bearing group 3 medulloblastoma significantly, from 21 days in control mice to >95 days (P ≤ .001).
Craniospinal irradiated mice developed leukopenia within 1 week of treatment that was fully reversible by 4 weeks after cessation of treatment. No significant changes in other blood counts, liver enzymes, or body weight were observed.
It was concluded that an efficient and reproducible method to deliver craniospinal irradiation to mouse models of patient-derived xenografts of high-risk medulloblastoma was developed.
Image-guided craniospinal irradiation up to 36 Gy appeared well tolerated and improved overall survival significantly, even in models of high-risk medulloblastoma.
Dr Roussel said “The ultimate goal of our implementation of cranial spinal irradiation in mice bearing patient-derived tumors was the development of a clinical pipeline that faithfully represents the current therapeutic regimen in children with high-risk medulloblastoma. This pipeline will include tumor resection, radiation therapy, chemotherapy, and targeted therapy eventually. We hope our work will facilitate translation to the clinic.”
Xstrahl Life Science systems are being used to develop pre-clinical treatments all over the world. Find out how the SARRP, and MuriPlan can help achieve your next clinical studies.
This Xstrahl In Action was adapted from a article found on a National Library of Medicine website.