The radiopharmaceutical (131)I-meta-iodobenzylguanidine ((131)I-MIBG) is an effective treatment for neuroblastoma. However, maximal therapeutic benefit from (131)I-MIBG is likely to be obtained by its combination with chemotherapy.
In a previous paper by Nile DL, Rae C, Hyndman IJ, Gaze MN, Mairs RJ it was established that enhanced antitumour efficacy of (131)I-MIBG by inhibition of the poly(ADP-ribose) polymerase-1 (PARP-1) DNA repair pathway using the phenanthridinone derivative PJ34. Recently developed alternative PARP-1 inhibitors have greater target specificity and are expected to be associated with reduced toxicity to normal tissue. Their new paper “An evaluation in vitro of PARP-1 inhibitors, rucaparib and olaparib, as radiosensitisers for the treatment of neuroblastoma.” intended to determine whether the more specific PARP-1 inhibitors rucaparib and olaparib enhanced the efficacy of X-radiation or (131)I-MIBG.
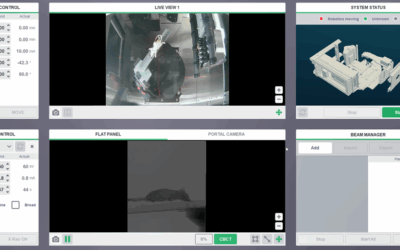
Radiosensitisation of SK-N-BE(2c) neuroblastoma cells or noradrenaline transporter gene-transfected glioma cells (UVW/NAT) were investigated using clonogenic assay. Propidium iodide staining and flow cytometry was used to analyse cell cycle progression. DNA damage was quantified by the phosphorylation of H2AX (γH2AX).
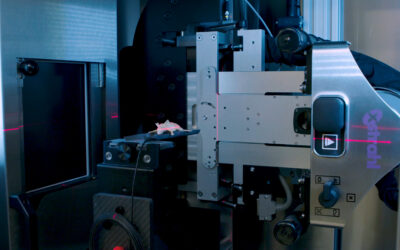
By combining PARP-1 inhibition with radiation treatment, using the Xstrahl SARRP, it was possible to reduce the X-radiation dose or (131)I-MIBG activity concentration required to achieve 50 % cell kill by approximately 50 %. Rucaparib and olaparib were equally effective inhibitors of PARP-1 activity. X-radiation-induced DNA damage was significantly increased 2 hours after irradiation by combination with PARP-1 inhibitors (10-fold greater DNA damage compared to untreated controls). Moreover, combination treatment prevented the restitution of DNA, exemplified by the persistence of 3-fold greater DNA damage after 24 hours, compared to untreated controls (p < 0.01) and induced greater G2/M arrest than either single agent alone.
It was therefore concluded that rucaparib and olaparib sensitise cancer cells to X-ray radiation or (131)I-MIBG treatment. It is likely that the mechanism of radiosensitisation entails the accumulation of unrepaired radiation-induced DNA damage. Our findings suggest that the administration of PARP-1 inhibitors and (131)I-MIBG to high risk neuroblastoma patients may be beneficial.
This Xstrahl In Action was adapted from a article found on a National Library of Medicine website.