Changes in cancer care are driving a demand for improved outcomes at greater efficiency and reduced costs. In this presentation, Dr. Julya Zuenkova describes a value-based approach in radiotherapy to use X-rays for operational efficacy. Learn how these treatments further advance patient adherence to treatment and improve skin cancer patients’ pathways for greater quality of life and shared decision making.
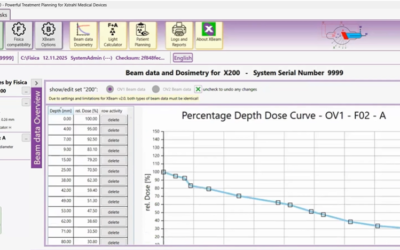
XBeam V2: FDA-Approved Treatment Planning Software
The Fastest, Safest Way to Plan Superficial & Orthovoltage Treatment XBeam V2 is the revolutionary, FDA 510(k)-cleared dose planning software designed to automate planning, eliminate risk, and seamlessly integrates with the Concerto system, Xstrahl’s...