In this video, Prof. Dr. med Bernadette Eberlein presents on using Radiotherapy for Peyronie Disease. This presentation was part of the 2021 clinical symposia, Radiotherapy for Benign Conditions. See the full recording at https://xstrahl.com/symposia-session-2/
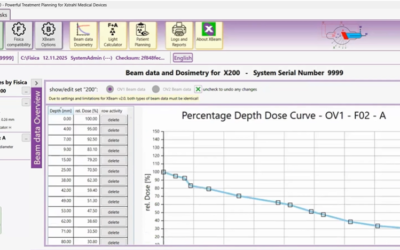
XBeam V2: FDA-Approved Treatment Planning Software
The Fastest, Safest Way to Plan Superficial & Orthovoltage Treatment XBeam V2 is the revolutionary, FDA 510(k)-cleared dose planning software designed to automate planning, eliminate risk, and seamlessly integrates with the Concerto system, Xstrahl’s...