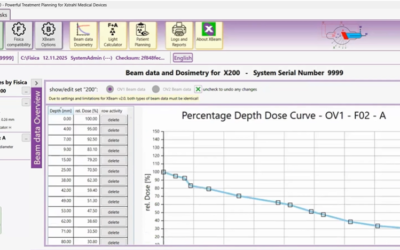
High-grade glioma (HGG), a deadly primary brain malignancy, manifests radioresistance mediated by cell-intrinsic and microenvironmental mechanisms. High levels of the cytokine transforming growth factor-β (TGF-β) in HGG promote radioresistance by enforcing an effective DNA damage response and supporting glioma stem cell self-renewal. Our analysis of HGG TCGA data and immunohistochemical staining of phosphorylated Smad2, which is the main transducer of canonical TGF-β signaling, indicated variable levels of TGF-β pathway activation across HGG tumors. These data suggest that evaluating the putative benefit of inhibiting TGF-β during radiotherapy requires personalized screening. Thus, we used explant cultures of seven HGG specimens as a rapid, patient-specific ex vivo platform to test the hypothesis that LY364947, a small molecule inhibitor of the TGF-β type I receptor, acts as a radiosensitizer in HGG. Immunofluorescence detection and image analysis of γ-H2AX foci, a marker of cellular recognition of radiation-induced DNA damage, and Sox2, a stem cell marker that increases post-radiation, indicated that LY364947 blocked these radiation responses in five of seven specimens. Collectively, our findings suggest that TGF-β signaling increases radioresistance in most, but not all, HGGs. We propose that short-term culture of HGG explants provides a flexible and rapid platform for screening context-dependent efficacy of radiosensitizing agents in patient-specific fashion. This time- and cost-effective approach could be used to personalize treatment plans in HGG patients.
N. Sumru Bayin, Lin Ma, Cheddhi Thomas, Rabaa Baitalmal, Akhila Sure, Kush Fansiwala, Mark Bustoros, John G. Golfinos, Donato Pacione, Matija Snuderl & David Zagzag