Treating skin cancers with surgery is still the standard of care for most NMSC patients. However, surgery is not without potential downsides, especially for patients with other health issues. Surface radiotherapy, both Electronic Brachytherapy (EBT) and Superficial Radiation Therapy (SRT), is an FDA-approved treatment option and an excellent choice for select NMSC patients. Explore the case study below to see how Dr. Steven Davis has had success treating patients with surface radiation therapy.
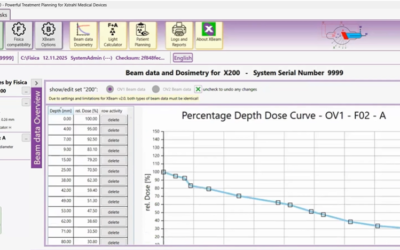
XBeam V2: FDA-Approved Treatment Planning Software
The Fastest, Safest Way to Plan Superficial & Orthovoltage Treatment XBeam V2 is the revolutionary, FDA 510(k)-cleared dose planning software designed to automate planning, eliminate risk, and seamlessly integrates with the Concerto system, Xstrahl’s...