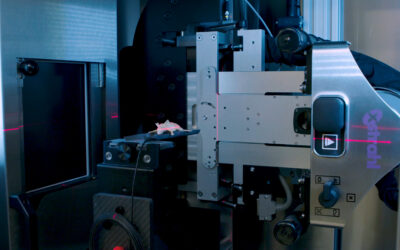
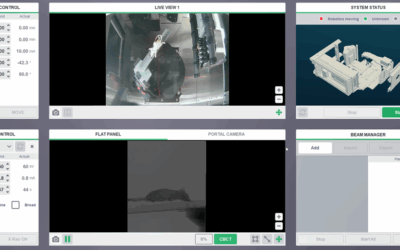
Resistance to radiotherapy due to insufficient cancer cell death is a significant cause of treatment failure in non-small cell lung cancer (NSCLC). The endogenous caspase-8 inhibitor FLIP is a critical regulator of cell death that is frequently overexpressed in NSCLC and is an established inhibitor of apoptotic cell death induced via the extrinsic death receptor pathway. Apoptosis induced by ionizing radiation (IR) has been considered to be mediated predominantly via the intrinsic apoptotic pathway; however, we found that IR-induced apoptosis was significantly attenuated in NSCLC cells when caspase-8 was depleted using RNA interference (RNAi), suggesting involvement of the extrinsic apoptosis pathway. Moreover, overexpression of wild-type FLIP, but not a mutant form that cannot bind the critical death receptor adaptor protein FADD, also attenuated IR-induced apoptosis, confirming the importance of the extrinsic apoptotic pathway as a determinant of response to IR in NSCLC. Importantly, when FLIP protein levels were downregulated by RNAi, IR-induced cell death was significantly enhanced. The clinically relevant histone deacetylase (HDAC) inhibitors vorinostat and entinostat were subsequently found to sensitize a subset of NSCLC cell lines to IR in a manner that was dependent on their ability to suppress FLIP expression and promote activation of caspase-8. Entinostat also enhanced the antitumor activity of IR in vivo Therefore, FLIP downregulation induced by HDAC inhibitors is a potential clinical strategy to radiosensitize NSCLC and thereby improve response to radiotherapy. Overall, this study provides the first evidence that pharmacological inhibition of FLIP may improve response of NCSLC to IR.
McLaughlin KA, Nemeth Z, Bradley CA, Humphreys L, Stasik I, Fenning C, Majkut J, Higgins C, Crawford N, Holohan C, Johnston PG, Harrison T, Hanna GG, Butterworth KT, Prise KM, Longley DB.