Radiation therapy, traditionally used to successfully treat many internal cancers, can also provide highly effective—and often superior—therapy for skin cancers. Electron radiation therapy, which uses high-energy rays, is the choice for safe, effective skin cancer treatment without the risks of surgery. Yet, today, few dermatologists routinely consider electrons as an alternative or complement to surgical treatment. In large part, this is because the logistics of electron radiation therapy delivery have been problematic due to linear accelerator equipment size and shielding requirements. Moreover, with therapy historically provided at free-standing oncology centers, dermatologists no longer remain part of the continuum of care, compromising both patient relationships and revenues.
Today, technology is changing that. New innovations—including an affordable, selfshielded, electron linear accelerator that does not require lead or concrete bunkers—are providing dermatology practices with cost-effective and practical options for delivering electron therapy for a wide variety of skin cancers.
As a standalone treatment or a complement to surgery, targeted electron radiation therapy provides an elegant alternative for difficult-to-treat skin cancer cases, from cosmetically challenging tumors to diabetic patients.
Most electron therapy applications have long enjoyed strong Medicare and private payer reimbursements. When implemented appropriately from a business and administrative standpoint, dermatology office-based electron radiation therapy requires a relatively low patient volume to break even and need not involve significant capital expense to dramatically enhance practice revenues.
With the advances in electron radiation therapy and recent cuts in Mohs surgery reimbursement, dermatologists would be well served to investigate the feasibility and benefits of incorporating office-based electrons into their practice and offering a multidisciplinary skin cancer treatment solution.
This white paper explores the benefits of electron radiation therapy, available electron technologies and logistics of incorporating them into a dermatology practice for enhanced patient care and satisfaction as well as practice revenues.
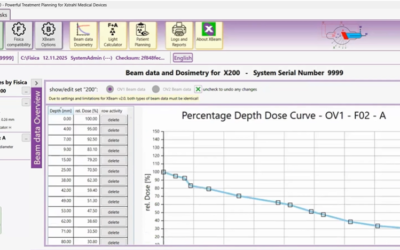
XBeam V2: FDA-Approved Treatment Planning Software
The Fastest, Safest Way to Plan Superficial & Orthovoltage Treatment XBeam V2 is the revolutionary, FDA 510(k)-cleared dose planning software designed to automate planning, eliminate risk, and seamlessly integrates with the Concerto system, Xstrahl’s...