Recently, Dr. Steven Davis shared his experience of using RADiant in Dermatology Times. See his piece below. Steven A Davis, M.D., is a board-certified dermatologist and Medical Director of the Dermatology & Laser Center of San Antonio.
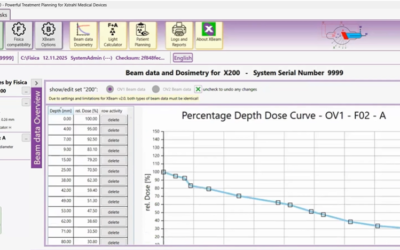
XBeam V2: FDA-Approved Treatment Planning Software
The Fastest, Safest Way to Plan Superficial & Orthovoltage Treatment XBeam V2 is the revolutionary, FDA 510(k)-cleared dose planning software designed to automate planning, eliminate risk, and seamlessly integrates with the Concerto system, Xstrahl’s...