The radiosensitizing efficacy of gold is well established, and has often been tested on Xstrahl systems, such as investigated in the Xstrahl in Action: Gold nanoparticles as dose-enhancement agent for X-ray therapy of melanomas however, there remain several significant barriers to the successful clinical translation of nano-sized gold particles (AuNPs).
These barriers include: retaining stability in relevant biological sera, demonstrating effectiveness at clinically relevant AuNP concentrations and identifying the biological context where significant benefit is most likely to be achieved.
Therefore in the paper “Unraveling the cell-type dependent radiosensitizing effects of gold through the development of a multifunctional gold nanoparticle” Nicol JR, Harrison E, O’Neill SM, Dixon D, McCarthy HO, Coulter JA, developed a AuNP preparation, stress-tested to provide effective protection from salt and serum mediated agglomeration. Furthermore, the core AuNP is co-functionalized with two biologically derived peptides designed to enhance endocytosis and promote endosomal escape, thus maximizing intracellular AuNP surface area.
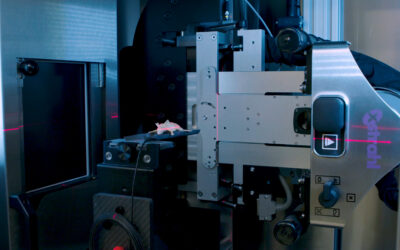
Their investigations demonstrated restored AuNP internalization using the co-functionalized preparation that generated significant radiosensitization, in both in vitro and in vivo models, tested on the Xstrahl Small Animal Radiation Research Platform, at clinically viable treatment concentrations. Furthermore, they have identified an underpinning biological mechanism in the inherent radical scavenging capacity that could be used to predict radiosensitizing efficacy.
This Xstrahl In Action was adapted from a article found on a National Library of Medicine website.